Abstract
Background: Accurate heart function estimation is vital for detecting and monitoring cardiovascular diseases. While two-dimensional echocardiography (2DE) is widely accessible and used, it requires specialized training, is prone to inter-observer variability, and lacks comprehensive three-dimensional (3D) information.
Aim: We introduce CardiacField, a computational echocardiography system using a 2DE probe for precise, automated left ventricular (LV) and right ventricular (RV) ejection fraction (EF) estimations, especially easy-to-use for non-cardiovascular health care practitioners. We assess the system's usability among amateur users and evaluate its performance against expert interpretations and advanced deep learning tools.
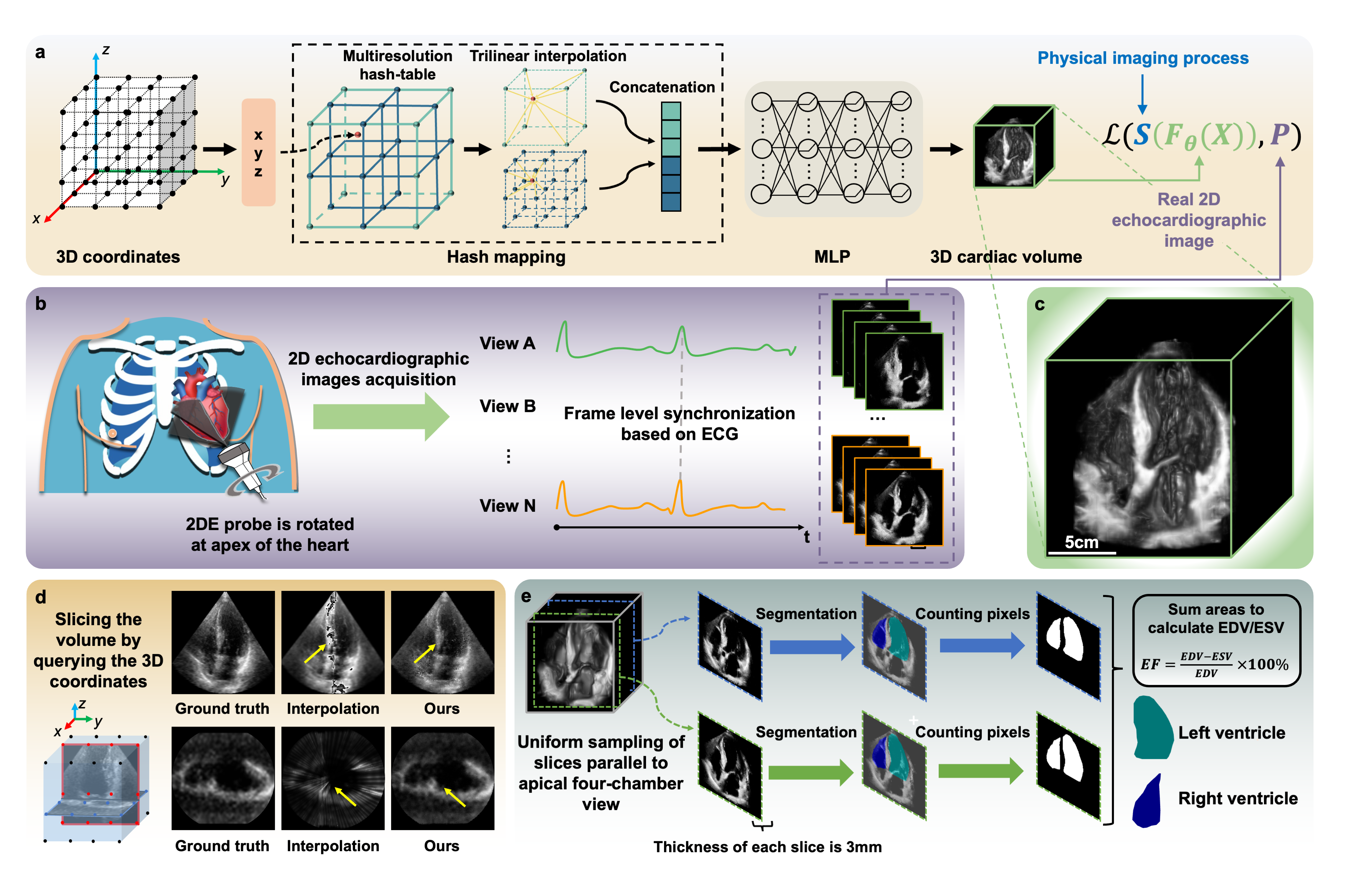
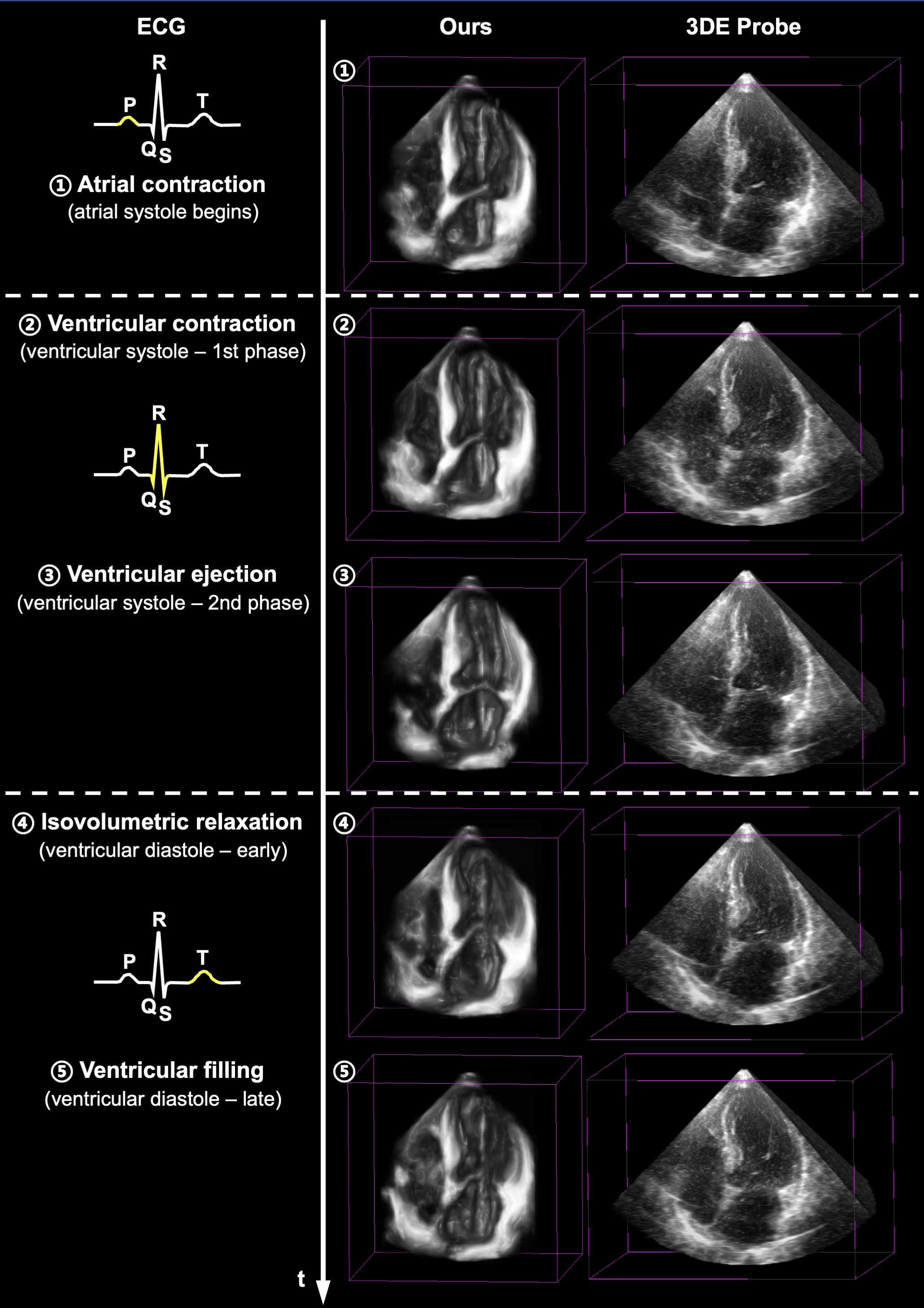
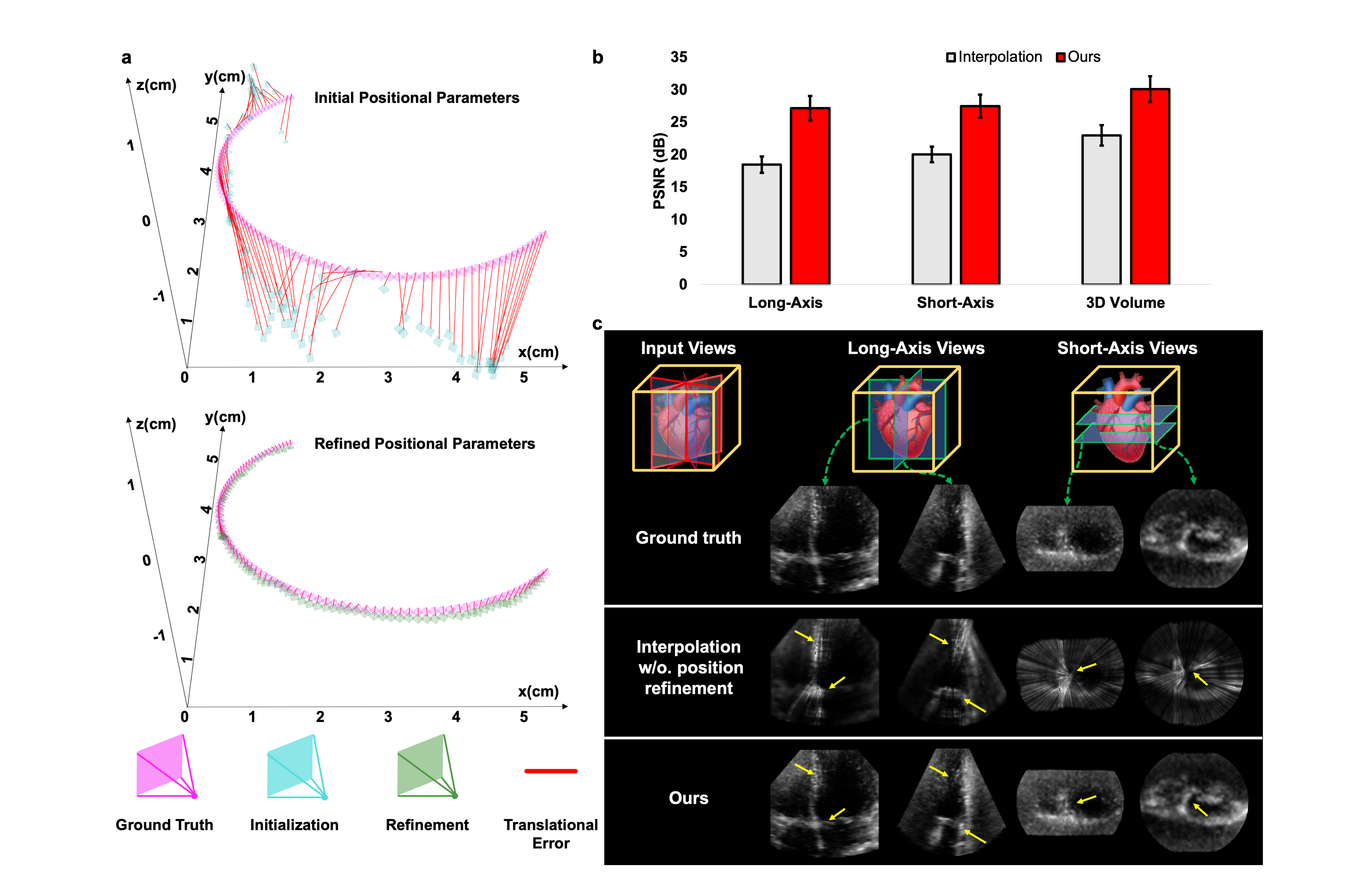
Methods: We developed an implicit neural representation network to reconstruct a 3D cardiac volume from sequential multi-view 2D echocardiographic images, followed by automatic segmentation of left ventricular (LV) and right ventricular (RV) areas to calculate volume sizes and ejection fraction (EF) values. Our study involved 127 patients to assess EF estimation accuracy against expert readings and 2D video-based deep learning models. A subset of 56 patients was utilized to evaluate image quality and 3D accuracy, and another 50 to test usability by amateurs and across various ultrasound machines.
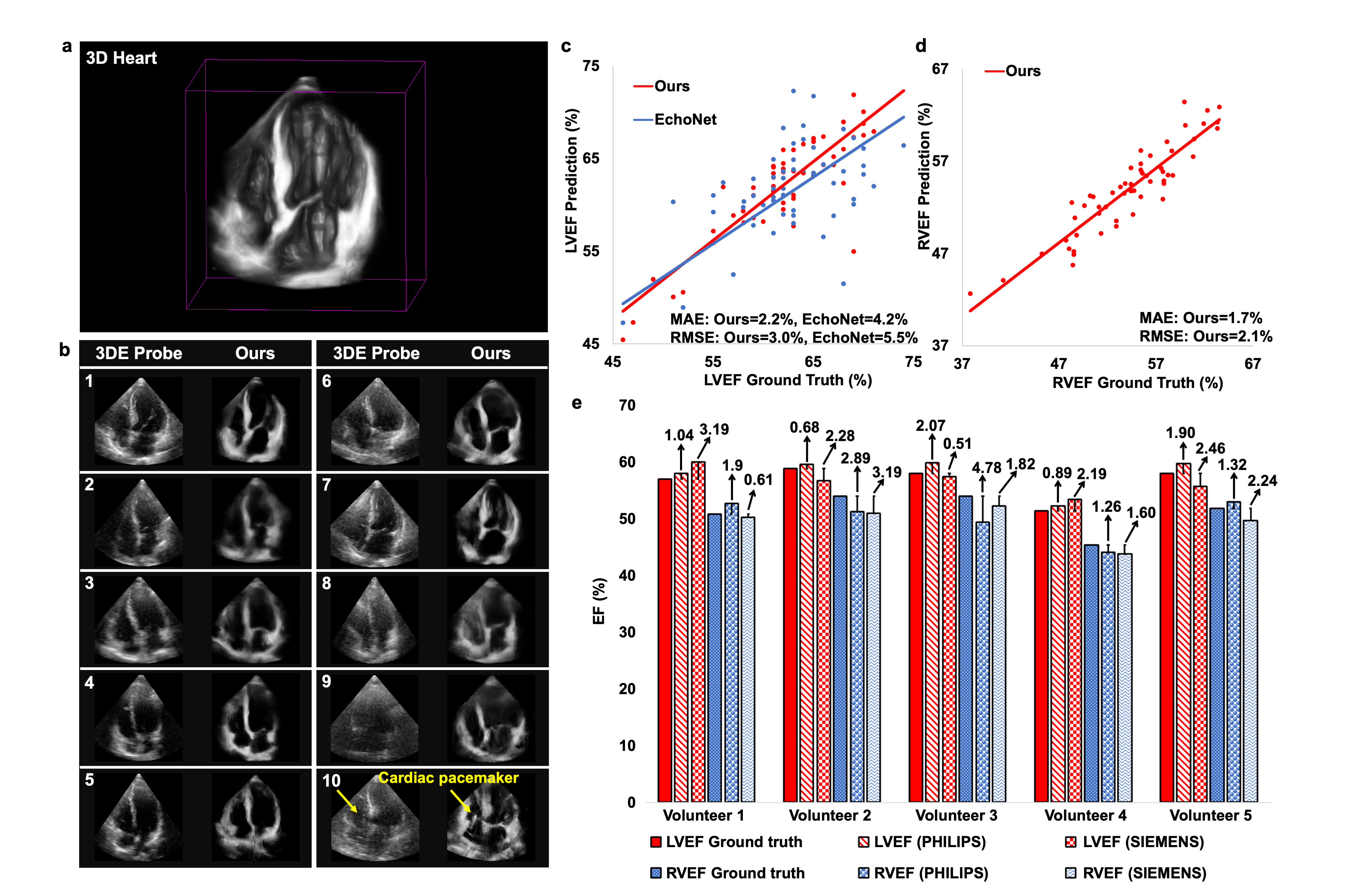
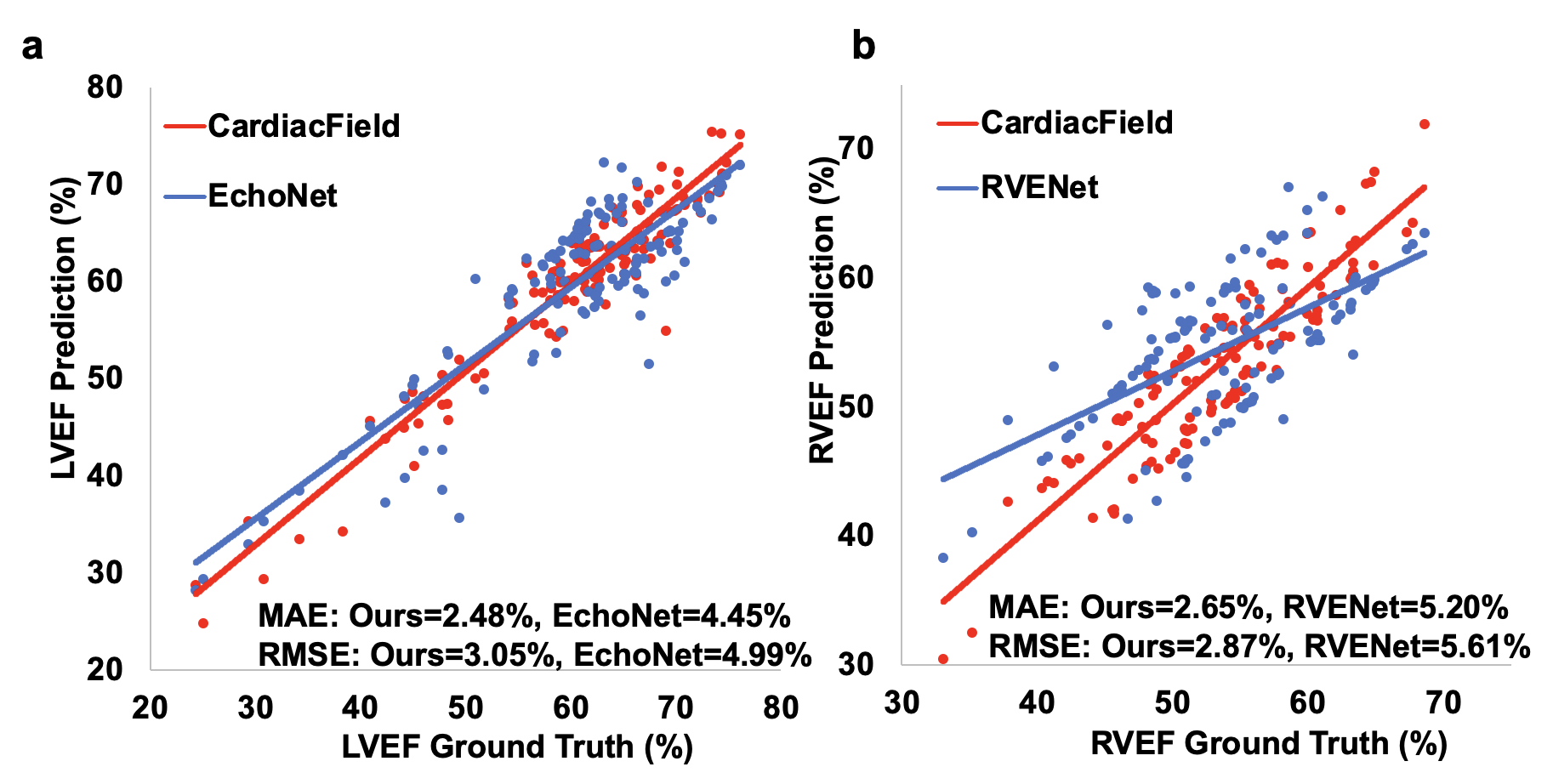
Results: CardiacField generates a 3D heart from 2D echocardiograms with <2 minute processing time. The LVEF predicted by our method has a mean absolute error (MAE) of 2.48%, while the RVEF has an MAE of 2.65%.Conclusions: Employing a straightforward apical ring scan with a cost-effective 2DE probe, our method achieves a level of EF accuracy for assessing LV and RV function that is comparable to that of 3DE probes.
The workflow of the CardiacField